zinc 2+ electron configuration|Iba pa : Clark What is the Electron Configuration of Zinc. [Ar] 3d10 4s2 is the electron configuration of Zinc. How Many Valence Electrons does Zinc Have. Zinc has two valence electrons in its outer shell. Chlorine .
The Crafting Nook is a resource for all things Home Decor, DIY and Craft ideas, Organization, Video Tutorials, Quick Recipes and so more.
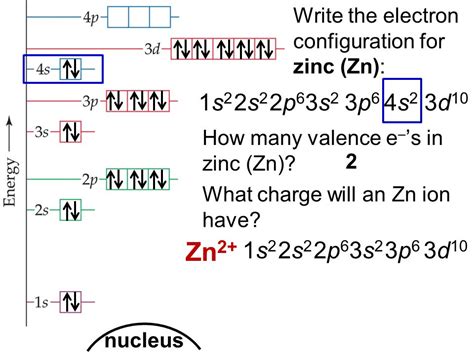
zinc 2+ electron configuration,101K views 3 years ago. To write the configuration for the Zinc and the Zinc ion, first we need to write the electron configuration for just Zinc (Zn). We first need to find the number of. Mar 23, 2023
The electronic configuration of Zinc is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Electronic configuration of Zinc ion Zn 2 +: Zn loses its two electrons and results in the formation of .
Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. Sm: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 .Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism.
What is the Electron Configuration of Zinc. [Ar] 3d10 4s2 is the electron configuration of Zinc. How Many Valence Electrons does Zinc Have. Zinc has two valence electrons in its outer shell. Chlorine . The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .What is the Electron Configuration for ZN2+? The given element is Zinc. The symbol of the element is Zn. Atomic number of zinc – 30. Electronic configuration – 1s 2 2s 2 2p 6 .
Zinc represents the symbol Zn in the periodic table. Let us discuss about electronic configuration of zinc in this article. The electronic configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10.Electrons are distributed among the shells K, L, M, and N. Zn is the 12 th group element and one of the transition metals in the periodic table.. We .
Full electron configuration of zinc: 1s2 2s2 2p6 3s2 3p6 3d10 4s2. copper ← zinc → gallium. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the .The electron configuration turns out to be 4s 2, 3d 1. It's actually 4s 2, 3d 1 or if you prefer 3d 1, 4s 2 once again with argon in front of it. . We're adding one more, writing one more electrons. We just took care of copper. For zinc we have one more electron and so you could think about this being 4s 2 right here and then we have 3d 10 .Dans le tableau périodique, les éléments sont classés par ordre croissant de numéro atomique Z. La configuration électronique du zinc est [Ar] 3d10 4s2. Les états d’oxydation possibles sont +2. Le zinc a une configuration électronique de [Ar]3d104s2 et fait partie du groupe 12 du tableau périodique. C’est un métal modérément .Iba paThe electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
Element Zinc (Zn), Group 12, Atomic Number 30, d-block, Mass 65.38. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .

The atomic number of zinc is $$30$$, which means that the neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have $$30$$ electrons. The electron configuration of a neutral zinc atom is $$1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}$$.
zinc 2+ electron configuration Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.The chemical symbol for Zinc is Zn. Electron Configuration and Oxidation States of Zinc. Electron configuration of Zinc is [Ar] 3d10 4s2. Possible oxidation states are +2. Electron Configuration. The periodic .

The atomic number of Zinc (Zn) is 30. Therefore, the electronic configuration of Zinc can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Here, the first shell contains 2 electrons (1s²), the second shell contains 8 electrons (2s² 2p⁶), the third shell contains 18 electrons (3s² 3p⁶), and the fourth shell contains 2 electrons in the s . The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .The atomic number of zinc is $$30$$, which means that the neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have $$30$$ electrons. The electron configuration of a neutral zinc atom is $$1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}$$.
Introduction to electron configurations. Google Classroom. About. Transcript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
zinc 2+ electron configuration Iba paIntroduction to electron configurations. Google Classroom. About. Transcript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.Zinc has an electron configuration of [Ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent. . When compounds in this oxidation state are formed, the outer shell s electrons are lost, yielding a bare zinc ion with the electronic configuration [Ar]3d 10.
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first.Zinc has an electron configuration of [Ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent. The surface of the pure metal tarnishes quickly, eventually forming a protective passivating layer of the basic zinc carbonate, Zn 5 (OH) 6 (CO 3)
zinc 2+ electron configuration|Iba pa
PH0 · how to write electron configuration
PH1 · electron configuration guide
PH2 · electron configuration for every element
PH3 · electron configuration chart
PH4 · electron configuration calculator
PH5 · Iba pa